





|
Overview:
Rolls has developed
investigations into the effective connectivity of the human cerebral
cortex complemented by functional connectivity and diffusion
tractography, utilising 360 cortical regions defined in the Human
Connectome Project Multimodal Parcellation (HCP-MMP) atlas (Glasser et
al 2016 Nature 536: 171-178; 645), data from the Human Connectome Project at 7T, and an
effective connectivity algorithm developed by Gustavo Deco (647, 656). These investigations provide great insight into cortical processing streams as described
next, and are making key contributions to understanding what
computations are performed by each brain region given its connectivity (B16).
Cortical visual streams (656, B16, 676, 682, 685, 688, 695)
A Ventrolateral Visual ‘What’
Stream for object and face recognition projects hierarchically to the
inferior temporal visual cortex which projects to the orbitofrontal
cortex for reward value and emotion, and to the hippocampal memory
system (656, B16, 682, 695). Visual task-related magnetoencephalography has been used to confirm the directionality in these visual cortical pathways (676).
A Ventromedial Cortical Visual Stream
has connectivity via the ventromedial visual areas to the parahippocampal scene (or
place) area that builds scenes utilising overlapping ventral visual
stream features, and thereby provides a ventral stream 'where' input to the
human hippocampus for episodic memory and navigation (656, 682, 695).
Visual task-related magnetoencephalography has been used to confirm the
directionality in this visual cortical pathway (688). The concept and connectivity are new and are leading towards a better
understanding of hippocampal function in memory and navigation in
primates including humans (662, 686, 692, 696, 702). A
key concept is that the 'where' spatial view representations in the
primate including human hippocampus are built by feature combinations,
then attractor networks and gain modulation by gaze in a ventromedial
visual cortical stream to the primate including human hippocampus, very
different from the place
representations in rodents (696, 692, 686, 662, 682, 370, 393).
A Dorsal Visual Stream
connects via V2 and V3A to MT+ Complex regions (including MT and MST),
which connect to intraparietal regions (including LIP, VIP and MIP)
involved in visual motion and actions in space. It performs coordinate
transforms for idiothetic update of Ventromedial Stream scene
representations (656,
662, 655,
612, 682, 696).
Connectivity has been discovered from inferior
parietal PGp (which receives from parietal area 7 regions) to the
hippocampus which is implicated in the self-motion (idiothetic) update
of parahippocampal and hippocampal spatial view cells using eye
position and head direction information (656,
655,
612, 662).
It has been discovered that an Inferior bank of the STS (superior
temporal sulcus) cortex Semantic Stream receives from the Ventrolateral
Visual Stream, from visual inferior parietal PGi, and from the
ventromedial-prefrontal cortex reward system and connects to language systems (656, 654).
It has been discovered that a Superior bank of the STS cortex
Semantic Stream receives visual inputs from the Inferior STS Visual
Stream, inferior parietal PGi, and STV, and auditory inputs from A5, is activated by face
expression, motion and vocalization, and is important in social
behaviour, and connects to language systems (656, 654, 682).
This research was performed with
Human Connectome Project 7T fMRI data, and the directionality has been
confirmed with magnetoencephalography data (676, 688).
These connectivity investigations
are strongly complemented by activations in a task-related fMRI
investigation with 956 HCP participants using the HCP-MMP atlas, with
faces, scenes, tools and body parts as the stimuli (685). This
investigation helps to add function to the different visual and other
including semantic, somatosensory, and auditory regions whose
connectivity has been analysed as described above.
Some of these advances and discoveries of the connectivity of visual
cortical pathways in humans and how they are connected to memory,
emotion, semantic, and language systems are described in Rolls (2024)
Two What, Two Where, Visual Cortical Streams in Humans (682).
Posterior parietal cortex (655, B16)
Intraparietal areas LIP, VIP,
MIP, and AIP have connectivity from early cortical visual regions, and
to visuomotor regions such as the frontal eye fields, consistent with
functions in eye saccades and tracking. Five superior parietal area 7
regions receive from similar areas and from the intraparietal areas,
but also receive somatosensory inputs and connect with premotor areas
including area 6, consistent with functions in performing actions to
reach for, grasp, and manipulate objects.
In the anterior inferior
parietal cortex, PFop, PFt and PFcm are mainly somatosensory, and PF in
addition receives visuo-motor and visual object information, and is
implicated in multimodal shape and body image representations.
In the posterior inferior
parietal cortex, PFm and PGs combine visuo-motor, visual object, and
reward input and connect with the hippocampal system. PGi in addition
provides a route to motion-related superior temporal sulcus regions
involved in social interactions. PGp has connectivity with
intraparietal regions involved in coordinate transforms and may be
involved in idiothetic update of hippocampal and parahippocampal cortex visual scene
representations (655, 662, 692).
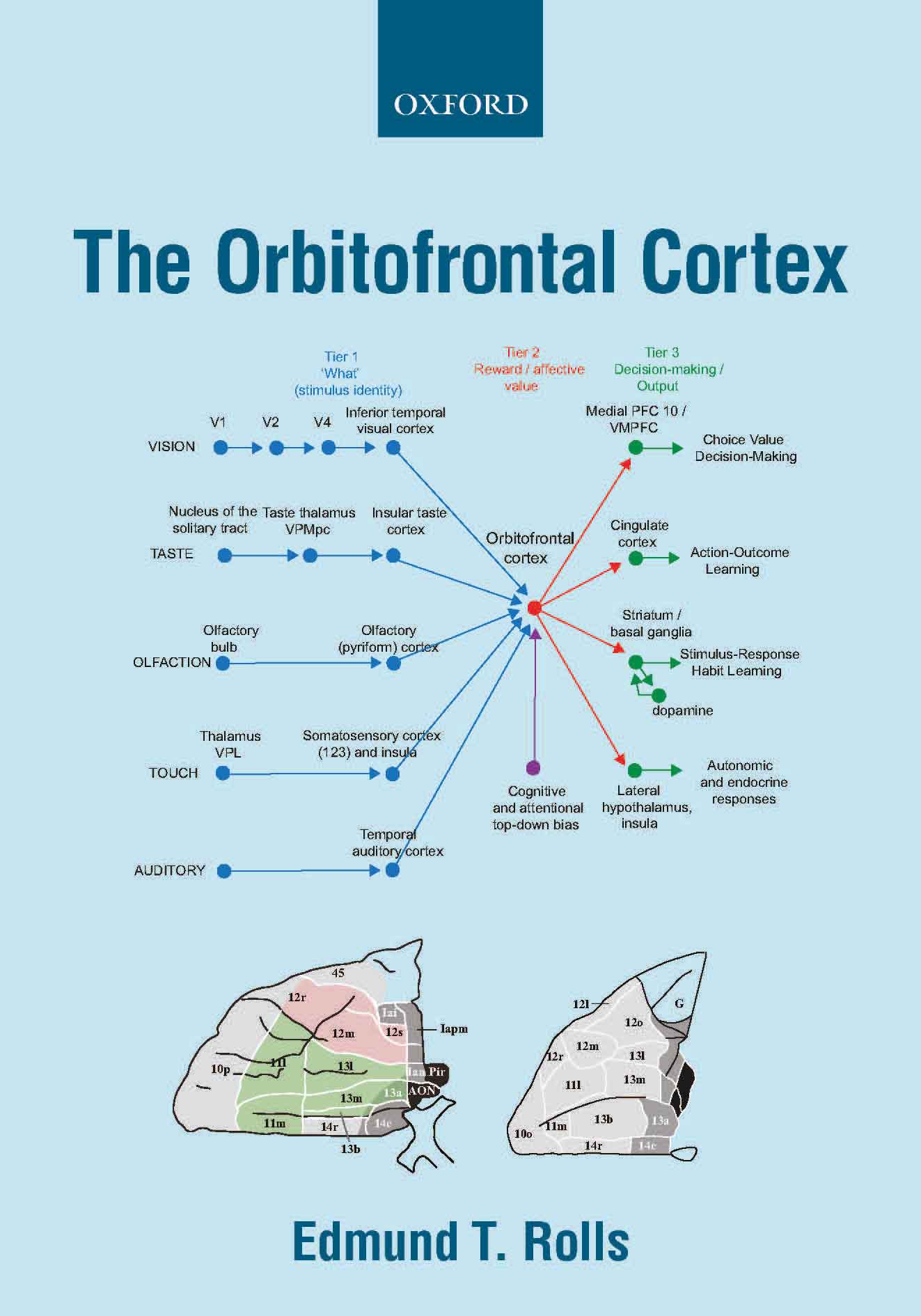
Orbitofrontal cortex, vmPFC, anterior cingulate cortex, and amygdala (649, 665, 657, B16, 674).
The orbitofrontal cortex has
effective connectivity from gustatory, olfactory, and temporal visual,
auditory and pole cortical areas. The orbitofrontal cortex has
connectivity to the pregenual anterior and posterior cingulate cortex
and hippocampal system, and provides for rewards to be used in memory
and navigation to goals.
The orbitofrontal and
pregenual anterior cortex have connectivity to the supracallosal
anterior cingulate cortex which projects to midcingulate and other
premotor cortical areas, and provide for action-outcome learning
including limb withdrawal or flight or fight to aversive and non-reward
stimuli.
The
lateral orbitofrontal cortex has outputs to language systems in the
inferior frontal gyrus and provides a route for declarative reports
about emotional states.
In contrast, the amygdala has
relatively little connectivity back to the neocortex in humans, and so
may be less involved in consciously experienced emotion than in
behavioural and autonomic responses to punishing and rewarding stimuli (665).
The medial orbitofrontal
cortex connects to the nucleus basalis of Meynert and the pregenual
anterior cingulate to the septum, and damage to these cortical regions may
contribute to memory impairments by disrupting cholinergic influences
on the neocortex and hippocampus normally involved in memory consolidation (649, 657).
Hippocampal systems for memory and navigation (657, 656, 655, 649, 647, 644, 635, 662, B16, 686, 692, 695).
The connectivity from the human
orbitofrontal cortex, vmPFC and anterior cingulate cortex to the
hippocampal system shows how reward value and emotion can reach the
hippocampal memory system to become incorporated into episodic memory (649, 657, 635, 644).
This also shows how these cortical regions have connectivity with the
septum and basal forebrain cholinergic systems, providing a mechanism
that may contribute to the memory impairments produced by vmPFC damage
in humans (649, 657).
These discoveries lead to a new approach to memory consolidation that
incorporates the roles of reward systems in memory consolidation (657).
The identification in humans using effective connectivity of a
ventromedial cortical visual stream via the ventromedial visual areas to the
parahippocampal scene (or place) area which builds scenes by
overlapping ventral visual stream features and thereby provides a ventral stream
'where' input to the hippocampus (656, 676, 688, 692, 695, 702). This concept and discovery is beyond anything known in rodents, and relates to spatial view cells (662, B16, 682, 686, 692, 702). Evidence that this cortical stream is selectively activated during episodic memory tasks involving spatial scenes vs words (690). A
key concept is that the 'where' spatial view representations in the
primate including human hippocampus are built by feature combinations,
then attractor networks and gain modulation by gaze in a ventromedial
visual cortical stream to the primate including human hippocampus (696, 692, 686, 662, 682, 370, 393).
In addition, I have shown how the hippocampal
episodic memory system has connectivity to anterior temporal lobe semantic
multimodal including visual regions, and to semantic inferior parietal visual
cortical regions, and have produced a theory and model of how the
hippocampal episodic memory inputs inputs could help to form
semantic memories (694).
The identification in humans of a pathway from inferior parietal
PGp which receives from parietal area 7 regions to the
hippocampus which is implicated in the self-motion (idiothetic) update
of parahippocampal and hippocampal spatial view cells using eye
position and head direction information (656, 655, 612). This is likely to be involved in navigation and memory in the dark and when the view details are obscured (662).
The identification in humans
of connectivity from the Ventrolateral Cortical Visual Stream to the lateral
temporal lobe for object and face representations via parahippocampal
TF to the hippocampus to provide 'what' information for the human
hippocampal memory system (656, 662, 676, 682, 695).
The identification in humans of the
connectivity of a transitional cortical area, the posterior cingulate
cortex (not present in rodents) between the neocortex and the
hippocampus (661).
A posterior part of the posterior cingulate regions has connectivity
with 'what' systems and the hippocampus, and an anterior part with
'where' systems, and both provide routes to and from the hippocampal
memory system (661, B16).
Cortical systems for language (654, B16)
A 'what and reward' semantic system
has been identified involving cortex in the ventral banks of the superior temporal sulcus,
the temporal pole,
inferior parietal PGi, and orbitofrontal cortex; and a visual face and
object motion and auditory semantic system in the dorsal bank of the superior
temporal temporal sulcus cortex especially implicated in social
semantics (654).
Both semantic systems have effective connectivity to Broca's area 44
and 45, which in humans has links to other nearby inferior frontal
cortex regions that are proposed to provide attractor networks for
syntactic computations (654, 537).
Auditory cortical pathways in humans (666, B16)
It has been possible to
follow the connectivity of the human auditory system from core auditory
cortex through belt and parabelt to A4, A5 and thereby to language
areas of the human cerebral cortex (666).
Somatosensory cortical pathways in humans (660, B16)
It has been possible to follow the
connectivity of the human somatosensory system from areas 3, 2 and 1 via
the opercular and frontal opercular cortical regions to the insula, and
thereby to the inferior parietal somatosensory regions PFop and PF, and
to show that PF also receives visual inputs making it not only the top
of the human somatosensory hierarchy, but also a multimodal region for
the representation of felt and grasped objects (660).
Prefrontal cortical regions for working memory and executive function in humans (660, B16)
It has been possible to show in humans
that inferior prefrontal regions have connectivity with the inferior
temporal visual cortex and orbitofrontal cortex, are implicated in
working memory for “what” processing streams, and provide connectivity
to language systems, including 44, 45, 47l, TPOJ1, and the superior
temporal visual area, for which it is proposed that they also provide
attractor networks (660, 654).
The dorsolateral prefrontal cortex regions that include area 46 have
connectivity with parietal area 7 and somatosensory inferior parietal
regions and are implicated in working memory for actions and planning.
The dorsal prefrontal regions, including 8Ad and 8Av, have connectivity
with visual regions of the inferior parietal cortex, including PGs and
PGi, and are implicated in visual and auditory top-down attention (660).
The frontal pole cortex, and a theory of its role in exploit vs explore (678)
The frontal pole cortex is
implicated in whether to exploit current rewards, or to switch
behaviour to explore for potentially better rewards. It has been
discovered that the frontal pole cortex, cortical area 10,
receives connectivity from the orbitofrontal and anterior cingulate
cortex reward/non-reward system, and has connectiivty with the dorsal
and dorsolateral prefrontal cortex executive control system. This leads
to a theory of how reward value and its modulation by sensory-specific
satiety act through the frontal pole cortex to influence whether or not
prefrontal executive control remain stable to continue exploitation of
rewards; or became unstable thereby facilitating switching to
explore other potential rewards (678).
|