|





|
Overview:
Rolls has developed a framework for understanding brain mechanisms of
emotion and motivation in primates including humans based on mechanisms
that represent
reward and punishment value in the orbitofrontal cortex but not at
earlier stages of cortical processing, and in which the orbitofrontal
cortex largely overshadows the amygdala. Rolls and colleagues
discovered expected and outcome reward and punishment value neurons,
and non-reward (negative reward prediction error) neurons in the
primate orbitofrontal cortex; and discovered how taste and olfactory
processing in primates occurs to produce reward value representations
of the sight, smell, taste and fat texture of food in the orbitofrontal
cortex that is important in understanding appetite and obesity. These
discoveries are complemented and supported by fMRI in humans, by
investigation
of emotional disorders that follow brain damage in humans (641), and by an
approach based on these foundations to understanding depression in
humans. Key summary descriptions are in B14, B11, B16, 674, 634, 626, 579, 698, and 558. A summary aimed for the general reader in the interests of the public dissemination of science is in B13. An account of how some of the discoveries were made is provided in section 2 of 692.
A
theory of emotion, motivation, pleasure, and reward; and the principles of
their
implementation in the brain (B5,
B11, B13, B14, B15, B16, 273, 520,
148, 364, 428, 509, 526, 531, 533,
534, 542, 552, 579, 674, 697).
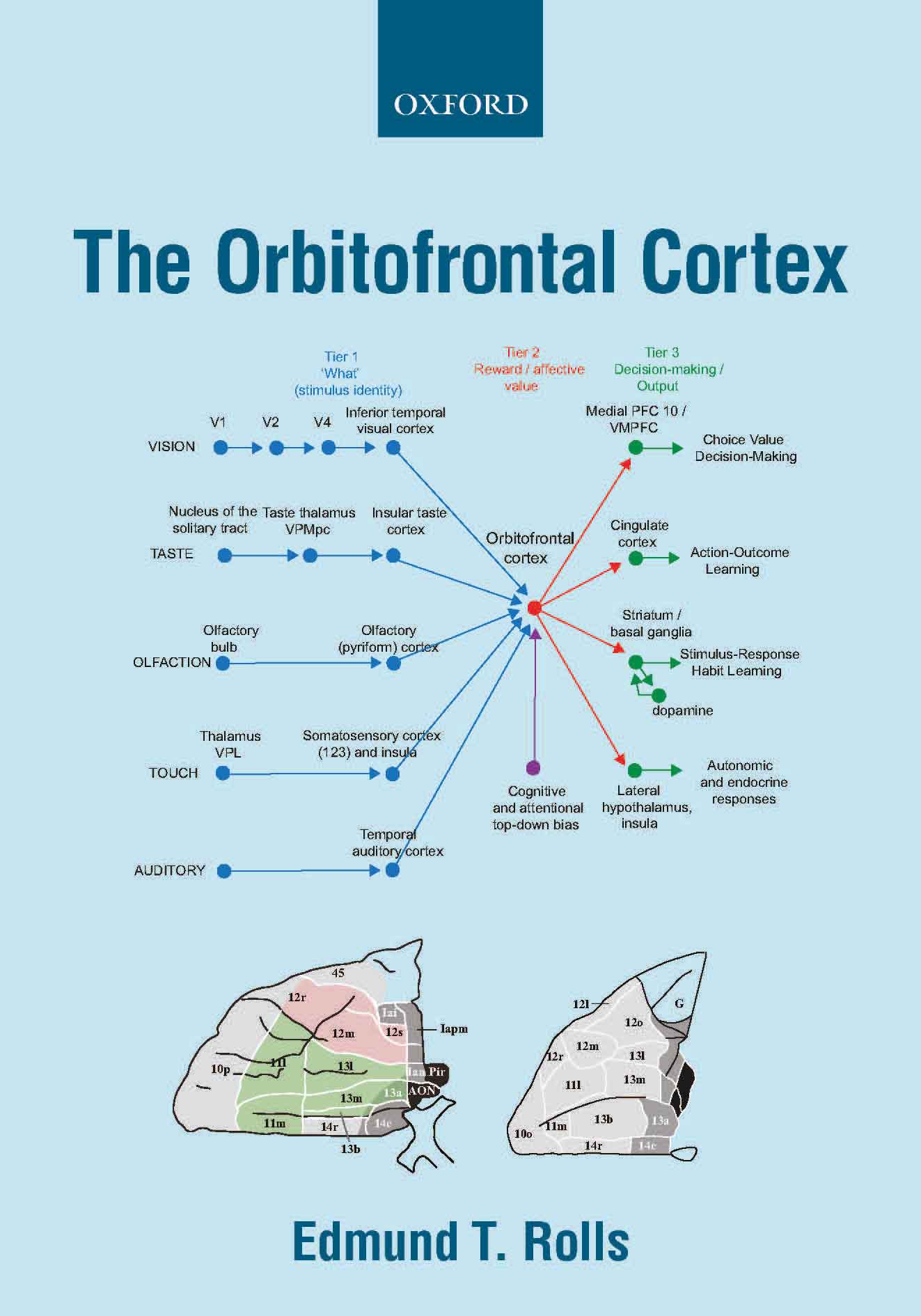
A key principle in primates
including humans is that reward value and emotional valence are
represented in the orbitofrontal cortex (and amygdala), whereas before
that in cortical processing, the representations are about objects and stimuli independently
of reward value, in the inferior temporal visual cortex, the primary taste
cortex in the insula, and in the olfactory cortex (B11, B13, B14, 674, 579, B15, B16, 698). This provides for the separation of emotion from perception. The
evidence from rodents is different, making them a poor model of emotion
and many other cortical systems in primates including humans (B16 Section 19.10). In humans, the reward-related and punisher-related represenations in the orbitofrontal
cortex are linearly correlated with subjectively experienced emotional
feelings of pleasure or unpleasantness.
In this framework, the dopamine
neurons are seen as receiving their information about reward and non-reward from brain regions such
as the orbitofrontal cortex, via the ventral striatum and habenula (572, B11, B13, B14, B16, 649), with connectional evidence in humans (649).
Further, orbitofrontal cortex neurons encode reward value and hence
emotion, independently of goal-related actions. The orbitofrontal
cortex provides reward-related information to the cingulate cortex for
action-outcome learning, and to the basal ganglia for habit-based
responses (B11, B13, B14, B16, 579, 606, 649, 674, 697).
A
theory of motivation, which considers motivations as states in which
rewards are being sought or punishers are being avoided, and emotions
as states elicited by receiving reward and punisment-related stimuli,
with the emotional states having motivational properties (557, 674, 697).
The discovery of lateral
hypothalamic neurons with visual and taste responses to food. These
neurons
only respond when hunger is present, so encode the reward value of
food. (25, 26. 27,
37, 57, B7,
B11).
Sensory-specific
satiety, discovered by recordings from lateral hypothalamic neurons
(46, 47, 55,
57, 59, 68, 69, 82, 89, 104, 234). Also known as reward-specific satiety, this is a key factor in how much food is eaten in a meal and is relevant to obesity (558).
Reward-specific satiety applies to almost all reward systems, and to no
pnishment system, and has adaptive value in locking behaviour to one
reward for some time, rather than continuously changing behaviour (674,
697). A synaptic mechanism for reward-specifc satiety and
reward-specific motivation (also known as incentive motivation) has
been described (700).
The discoveries that taste, olfactory, flavour, and visual sensory-specific satiety are implemented in
the
primate including human orbitofrontal cortex (124, 216, 285, 334).
The discovery of a basis
for brain-stimulation reward: activation of neurons normally activated
by
natural rewards (12, 48, B1,
B7).
The
discoveries that the primary taste cortex (119, 120, 437, 552),
the primary olfactory
cortex (442),
and the inferior
temporal visual cortex (32) encode information about the
identity and intensity
of the taste, odour or sight of objects, but not about their reward value (B11, 579, B16).
This is the case for primates and humans, but not for rodents which
provide a poor model of processing in these systems of primates
including humans (B14, B16).
The discovery of the
secondary taste cortex (in the primate orbitofrontal cortex, including lateral
and
medial parts) (124, 141,
190,
382).
Discovery of the
tertiary taste cortex (in the anterior cingulate cortex) (443,
468).
The discovery of multimodal
convergence of taste, olfactory and visual inputs onto single neurons
in the
orbitofrontal cortex to produce flavour (189,
366, 382)
using associative
learning for example between the sight and smell of food with its taste (211,
212). Age differences in this flavor reward system (544).
The discoveries that the
first cortical region in which information about reward value is made
explicit
in the representation in primates including humans is the orbitofrontal cortex (124, 291,
322,
333, 367,
441,
495, 579, B11, B14, B16, 674), and a
second is the anterior
cingulate cortex (443,
468, 495, B14, B16, 606, 674).
The evidence for reward value representations comes from rapid visual
reward reversal learning, and from devaluation by feeding to satiety (79, 212, 216, 674, B11, B16, 674).
Discoveries of the
roles of sensory-specific satiety, variety in the food available, and
top-down
cognitive and attentional control reward representations in the
orbitofrontal
cortex in appetite control and obesity, with implications for the prevention and control of obesity (416,
420,
426,
484,
487, 519, 542, B11, 558, 634, 638, 674, 700).
Discoveries of brain
regions where activity is associated with the subjective conscious
feeling of pleasure,
including the orbitofrontal cortex and anterior cingulate cortex (334,
335, 338,
434, 462, 495, 542, 544, B11, B13, B14, B16, 674).
The discovery that the
orbitofrontal cortex is involved in one-trial rule-based ('model-based') visual stimulus-reward
learning,
and contains negative reward prediction error neurons (79,
337, 579, 627,
B11, B13, B14, 674).
The discovery that the lateral orbitofrontal cortex is activated during reward
reversal and behavioural inhibition, when behaviour must be changed (337, 575, 627, B13, B14, B16, 626, 627, 674).
The discoveries that oral
texture, including viscosity, astringency, and fat texture, and oral
temperature, are represented in the primate including human primary taste cortex, the
orbitofrontal
cortex, and the amygdala (210, 346,
352,
361, 363,
376, 425, 499,
528, 542, 593, 610).
The discovery that fat is
sensed by its texture in the mouth (269,
336,
352,
363, 472, 475, 499).
The fat sensing is by the coefficient of sliding friction, not by
viscosity or by free fatty acids which are separately represented (593).This
has important implications for the development of foods with a similar texture,
but low
energy content (593, 610).
The
discovery that the top-down
control of reward value and emotion by cognition and attention is
implemented by modulation of responsiveness to stimuli in the
orbitofrontal cortex and anterior cingulate cortex (381,
434, 437,
440,
442,
480,
488, 520, 530, 606, B11, 674).
The discovery that synergism
between the taste of monosodium glutamate and consonant vegetable
odours produces the rich delicious flavour umami (158,
243,
279,
330, 417,
469).
The
representation of economic value in the orbitofrontal cortex, with
different
regions responding to monetary gains and losses (288,
424, 623, 627),
and both absolute
and relative value represented (467)
(see 495, B11, B14), with implications for economics (600).
The identification of reward-related
decision-making in the ventromedial prefrontal cortex / medial prefrontal cortex area 10, and the
representation
of confidence about decisions, based on an attractor network model of decision-making (452,
454, 481, 486, 489, 495, 513, B9, B11, B14, B16).
The discoveries of the
principles of operation of the orbitofrontal cortex in humans and other
primates (270,
356,
389,
357,
435, 452,
474,
478, 494, 495, 531, 579, 608, B7, B11, B13, B14, B16, 674, 698).
The connections of the orbitofrontal cortex that help it to implement its functions (190, 608, 620, 649, 665, B16, 674). One such discovery is of the effective connectivity of the human
orbitofrontal cortex, vmPFC and anterior cingulate cortex, which shows
how reward value and emotion can reach the hippocampal memory system to
become incorporated in episodic memory (649). This also shows how these
cortical regions have connectivity with the septum and basal forebrain
cholinergic systems, providing a mechanism that may contribute to the
memory impairments produced by vmPFC damage in humans (649, 657), and to the
effects of deep brain stimulation in these cortical regions used to
treat depression (649, 679).
The discovery that the lateral
orbitofrontal cortex has outputs to language systems in the inferior
frontal gyrus and provides a route for declarative reports about
emotional states (649, 665).
The discovery that, in contrast, the amygdala has relatively little
connectivity back to the neocortex in humans, and so may be less
involved in consciously experienced emotion in humans than in
behavioural and autonomic responses to punishing and rewarding stimuli (665).
The discoveries of face-selective
neurons in the amygdala (38, 91, 97), inferior temporal visual cortex (38A,
73,
91,
96, 162), and orbitofrontal cortex (397)
(see 412, 451, 501, 578, B11, B12, B14, B16).
The discoveries of face
expression selective neurons in the cortex in the superior temporal
sulcus (114,
126, 682) and orbitofrontal cortex (397). The discovery of reduced functional and effective connectivity in this region in autism (541, 570, 609). The use of effective connectivity to follow these cortical streams for face-related processing in humans (656, 676),
and adding function to the connectivity maps by analysing activations
to faces in 956 participants with the HCP-MMP atlas (685), and showing that these are left-lateralised especially in females.
Impairments
in the rapid rule-based reversal of associations between stimuli and reward value
in
patients with selective lesions of the orbitofrontal cortex and related
areas and
their relation to emotional changes (188,
203,
331,
354, 641). Also, impairments in
impulsivity (353,
362,
394).
These discoveries were
inspired by the discoveries
on neuronal activity in the orbitofrontal cortex, and are relevant to
understanding
the changes in patients with frontal lobe damage and in patients with
borderline personality disorder (B14).
A non-reward attractor theory of depression (559, 572, B13, 626, 679),
supported by altered connectivity and activations of the orbitofrontal
cortex in depression (564, 579, 583, 588, 591, 592, 596, 623, 626, B14, B16, 679), and a model of the computation of non-reward in
the orbitofrontal cortex (562). An introduction to the theory is available as a lecture.
The discoveries that the
subjective pleasantness of touch and unpleasantness of pain are
represented in the orbitofrontal cortex (322);
that the top-down cognitive effects on the pleasantness of touch and
the sight of touch are represented in the orbitofrontal and anterior
cingulate cortex (440);
and that in contrast to the somatosensory cortex, the insula in the
ventral somatosensory cortical stream (660) is not activated by the
sight of touch, implicating the insula in the representation of touch
to one's own body (440, 660).
The
roles of the cingulate cortex in emotion, action, and memory, and their
disorders, together with the concept that there is no single limbic
system (B11, B16, 531, 588, 596, 606, 608, 649, 657, 674).
The pregenual anterior cingulate cortex receives reward-related
information from the orbitofrontal cortex, and connects to the
hippocampal system to provide the value part of episodic memory and the
goals for navigation (649, 657).
A model for action-outcome learning in which action-related information
reaches the posterior cingulate cortex from the parietal cortex, and
reaches the supracallosal (dorsal) anterior cingulate cortex (649) from premotor regions as
well as posterior cingulate regions, is
associated in the supracallosal anterior cingulate cortex with reward
outcome information reaching there from the pregenual anterior
cingulate cortex and from the orbitofrontal cortex, with outputs
connecting to the
midcingulate cortex and the premotor cortex (606, 649, B16).
Consistent with this, human sensation-seeking (619), risk-taking (648) and body weight (638) relate to high connectivity between the reward-related medial orbitofrontal cortex and
the anterior cingulate cortex.
Basal
ganglia: each part of the striatum contains neurons that respond to
information
received from the cortical areas that project into each striatal
region, but
this information is brought together by the architecture of the
striatum,
globus pallidus, and substantia nigra in a way that appears to provide
for
selection of one behavioural output as a result of competition between
mutually
inhibitory neurons in these parts of the basal ganglia (80, 84, 147, 174, 181, B7,
B11, B16).
Extensions
of Granger causality and their application to functional neuroimaging:
componential Granger causality (which allows the effects of
interactions to be
measured); and Granger causality with signal-dependent noise (with
J.Feng, T.Ge
and colleagues) (505, 530). The use of effective (directed) connectivity in large-scale analyses of the neural bases of depression (583, 679), schizophrenia (602), and autism (609). The application of effective connectivity measured in the human brain to understand brain systems involved in emotion (649, 665); the roles of reward value in emotion and in memory (647, 649, 657);
and in somatosensation including the insula identified as part of a
ventral somatosensory stream in humans reaching the inferior parietal
cortex (660) with its inputs from the orbitofrontal cortex
implicating the insula in affective touch representations (660).
In
relation to addiction, the medial orbitofrontal cortex reward system
has high functional connectivity in those who tend to drink alcohol and
who are sensation-seekers and are impulsive, and the lateral
orbitofrontal cortex non-reward system has low functional connectivity
in those who tend to smoke and are impulsive (599). The medial orbitofrontal cortex is also activated by amphetamine (367), and has high functional connectivity in users of arecoline (betel quid) (617).
Reward systems and aesthetics
(B10, B11, 509,
532, 556, 574).
|